In the fast-paced arena of research and development (R&D), where the pursuit of innovation is a driving force, the landscape is undergoing a transformative period. With expanding industries and heightened global competition among manufacturers, the crux of competitive advantage now resides in the ability of organizations to innovate with efficiency and timeliness. As organizations grapple…
PFAS — the collateral damage begins
I predicted collateral damage as regulators addressed PFAS. I was right. On December 1, the EPA effectively killed Inhance Technologies’ barrier packaging business. The press on the EPA action is pretty bifurcated. Most are taking a victory lap, cheering that a producer of PFAS will cease production. A minority ask what will replace the technology…
Sustainable Ion Exchange Resin for Ultrapure Water Treatment, R&D 100 winner of the day
A critical component of the chip manufacturing process is the ultrapure water that is used in a range of process steps, such as wafer cleaning, that are highly sensitive to impurities in this process stream. H2O2 present in the UPW as a side product of UV treatment of organic impurities can cause problems such as…
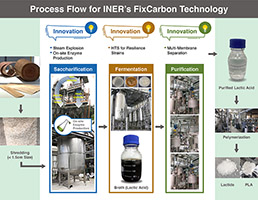
FixCarbon Technology: Carbon-negative bioplastics from afforestation, R&D 100 winner of the day
Developer: Institute of Nuclear Energy Research (INER), Atomic Energy Council, Executive Yuan, ROC (Taiwan) Co-Developer: NZ Bio Forestry, Inspira Applied Bio Solutions Co. FixCarbon technology revolutionizes the use of forestry waste and enables large-scale bio-plastic production using leftover Pinus Radiata wood processing and forestry waste as feedstock. The technology uses an acid-catalyzed steam explosion as…
MaterialsZone partners with Kafrit Group, enhancing products and customer experience
MaterialsZone, a materials Informatics platform for manufacturers’ R&D teams, and Kafrit Group, a global masterbatches and compounds producer, announced its long-term commercial agreement enabling Kafrit Group to better use its data to develop improved products and provide an unparalleled customer experience to its clients. With a crowded, competitive landscape, constantly changing customer demands, supply chain…
Dow Packaging Innovation Awards returns for its 2023/2024 edition
Dow is back with its 2023/2024 edition of its Packaging Innovation Awards (PIA), the packaging industry’s premier accolade for recognizing breakthroughs in technological advancement, sustainability, and enhanced user experience. As one of the industry’s longest-running independently judged awards, the global program — now into its 35th edition — will be held in Asia-Pacific this year…
R&D 100 winner of the day: DuPont BETATECH Thermal Interface Material
The automotive sector is changing at an unprecedented rate. This evolving landscape is being driven by legislation for reduced emissions, increasing urbanization, customer demand, and the need to reach price parity with internal combustion engine vehicles. With EV adoption expected to increase exponentially over the next few decades, OEM and tier suppliers are increasingly shifting…
Automakers to increase U.S. EV charging sites, in this week’s R&D Power Index
The R&D World Index (RDWI) for the week ending July 28, 2023, closed at 2,988.19 for the 25 companies in the RDWI. The Index was up 2.32% (or 30.57 basis points). Thirteen of the 25 RDWI members gained value last week from 2.03% (Apple Computer) to 11.99% (Stellantis NV). Twelve of the 25 RDWI…
R&D 100 winner of the day: Battelle’s Process for Converting Coal to High-Value Polyurethane Products
With rapidly growing U.S. demand for polyurethane (PU) products, especially foam products, we will remain heavily dependent on imported oil and thus be affected by worldwide disruptions to oil supply. To reduce this dependence on oil, we must investigate domestic feedstocks such as coal for creating PU products. Battelle’s breakthrough Coal-to-PU Foam process addresses this…
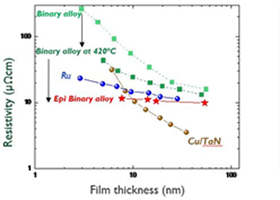
imec demonstrates conductor films on 300 mm wafers with low resistivity
This month, at the 2023 IEEE International Interconnect Technology Conference (2023 IEEE IITC), imec, a research and innovation hub in nanoelectronics and digital technologies, provides the first experimental evidence that the resistivity of a thin conductor film on a 300 mm Si wafer can be lower than that of Cu and Ru, which are currently…
R&D 100 winner of the day: Abcite 2060 Thermoplastic Flame Spray Powder Coating
Axalta Coating Systems’s Abcite 2060 is a breakthrough powder coating technology using innovative resin chemistry bringing unparalleled benefits. Foremost, Abcite 2060 powder coating offers sustainability benefits over incumbent liquid coating technology, containing no hazardous materials or VOCs. Abcite 2060 powder coating offers ease of application as it is applied in one layer on location without…
Bostik and Polytec PT launch new TCA range to spur innovation in battery design
Bostik and Polytec PT have collarborated to launch a new range of thermal conductive adhesives (TCA) to address the challenge of thermal management in the latest Cell-to-Pack (CTP) battery design for e-mobility solutions. Thermal management is vital to ensuring the operating temperature of EV-battery systems remains between 20° C and 40° C for optimum battery…
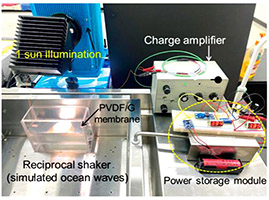
R&D 100 winner of the day: Black Magic Carpet for Water and Power Production (BMC-WP)
Developer: Advanced Membrane Materials Research Center (AMMRC), National Taiwan University of Science and Technology Co-Developer: King Membrane Energy Technology Inc. The Black Magic Carpet for Water and Power Production is the first innovation that combines simultaneous steam and power generation using an intelligent membrane with dual capabilities. PVDF/graphene membrane is used in the application of…
R&D 100 winner of the day: PPG COPPER ARMOR Anti-Viral and Anti-Bacterial Paint
PPG COPPER ARMOR is an antimicrobial paint that uses Corning Guardiant copper technology, a biocide comprised of copper glass ceramic particles, to continuously kill viruses and bacteria on the painted surface, including Staph, MRSA, E Coli, and COVID-19, within two hours of exposure for up to five years. The product is low-odor and has zero…
R&D 100 winner of the day: MAINCOTE HG-300 Emulsion for high performance one component metal protective coatings
There is a strong environmental driver for metal coatings transition from SB to WB systems. Although WB coatings have been successful in many applications, the use of WB coatings in more demanding environments with “medium corrosivity” have encountered notable challenges. After years of careful innovation, Dow Inc.’s MAINCOTE HG-300 Emulsion was developed with a distinct…
Smart material prototype challenges Newton’s laws of motion
From the Univerity of Missouri For more than 10 years, Guoliang Huang, the Huber and Helen Croft Chair in Engineering at the University of Missouri, has been investigating the unconventional properties of “metamaterials” — an artificial material that exhibits properties not commonly found in nature as defined by Newton’s laws of motion — in his…
R&D 100 winner of the day: Digital M functional resin material: Drops on where functionality is needed upon fabric
Taiwan Textile Research Institute Co-developer: Sabrina Fashion Industrial Corp., Fu Hsun Fiber Industries Co., Ltd. Digital M is a polyol polyurethane resin material with the function of moisture swelling and shrinking. When this material encounters moisture, it will produce a moisture swelling shrinkage effect, to create an “airflow channel” on the fabric surface. The channel…
What are spectrophotometers?
A spectrophotometer is an instrument used to determine the chemical composition of a sample by measuring how light at different frequencies passes through it. The spectrophotometer includes a light source to pass light through the sample, and a photodetector to measure the intensity of light passing through the sample. Most commonly the light is in…
What is fluorescence spectroscopy?
Fluorescence spectroscopy is a method of determining the composition of a sample. It excites a sample with electromagnetic radiation, causing it to emit characteristic radiation. This is a non-destructive method of analyzing sample composition. Instruments used to perform fluorescence spectroscopy are known as fluorometers. Most commonly the sample is excited using ultraviolet light and the…
What are elemental analyzers?
An elemental analyzer is an instrument that can determine the elemental composition of a sample. The analyzer may simply determine which elements are present, or it may make a quantitative analysis to identify how much of each element is present. In some cases, isotopic composition may also be determined. Elemental analyzers are used in many…
R&D 100 winner of the day: Low GWP Froth-Pak Spray Foam
DuPont’s Froth-Pak Spray Foam products are used to air seal and insulate various spaces within the building envelope. These products historically use hydrofluorocarbons as blowing agents which are being phased out of use in spray foam products because of their high global warming potential (GWP). Hydrofluoroolefin (HFO) blowing agents have been proposed as acceptable alternatives.…
R&D 100 winner of the day: LNP THERMOCOMP OFC08V compound
Chemical company SABIC introduced LNP THERMOCOMP OFC08V compound, the world’s first laser direct structuring (LDS) material solution for 5G base station dipole antennas and adjacent electrical/electronic applications. This new compound triggered the adoption and acceleration of novel plastic-based design in the 5G infrastructure industry. Technically, LNP THERMOCOMP OFC08V compound solved the hard problems of robust…
IACMI receives funding renewal from the DOE to continue composite R&D
The Institute for Advanced Composites Manufacturing Innovation (IACMI), headquartered in Knoxville, Tennessee, has announced it is receiving a funding renewal from the Department of Energy (DOE). IACMI becomes the first clean energy institute to be renewed by DOE. IACMI will be receiving federal funding across five fiscal years, with a first-year investment of $6 million…
R&D 100 of the day: More sustainable Collation Shrink Film enabled by REVOLOOP and DOWLEX GM AX01
Dow Packaging and Specialty Plastics, a business unit of Dow Inc. Co-developer: Polyrafia SA de CV Using its unique materials science capabilities and appropriately connecting two different technologies, REVOLOOP and DOWLEX GMAX01, Dow developed a secondary packaging with an enhanced sustainability approach, exceptional overall performance, and suitable shrinkage, making the mechanical properties of the packaging…
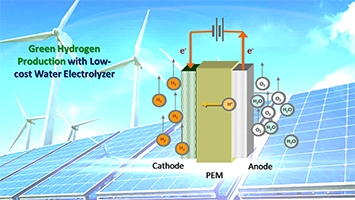
R&D 100 of the day: PGM-free OER Catalyst as Replacement of Iridium for PEM Water Electrolyzer
Argonne National Laboratory’s PGM-free OER catalysts derived from electrospun metal-organic framework demonstrated unprecedented activity and durability compared to other PGM-free materials, with the performance approaching that of commercial Ir. The new catalysts are operable in both acidic and alkaline media. It was incorporated into an operating proton exchange membrane electrolyzer and delivered a very high…